
We’ve rounded up some of the latest news and announcements pertaining to the coronavirus and the pharmaceutical industry. News from Health Hero, Prescryptive Health, FDA, AdhereHealth, AHIP, Novavax, medQ Inc., Alveo Technologies, DeliverHealth Solutions, HHS, Mayo Clinic and more.

We’ve rounded up some of the latest news and announcements pertaining to the coronavirus and the pharmaceutical industry. News from Terazo, Previon, Mobile Healthcare Services, Experity, CityMD, FDA, Direct Biologics, Armanino LLP, All Life Transitions and more.

We’ve rounded up some of the latest news and announcements pertaining to the coronavirus and the pharmaceutical industry. News from UPI, Updox, Allscripts, AMA, FDA, Kaiser Family Foundation, STAT, HHS, CDC, NIH and more.

We’ve rounded up some of the latest news and announcements pertaining to the coronavirus and the pharmaceutical industry. News from Moderna, Nitric Oxide Innovations, QuVa Pharma, Pfizer, AstraZeneca, The Doc, Apollo Medco, ThermoGenesis Holdings, Johnson and Johnson, FDA and more.

On Tuesday, June 30, 2020, the Senate Health, Education, Labor and Pensions committee will hold a hearing on the federal government’s ongoing response to COVID-19.

The U.S. Food and Drug Administration approved the FoundationOne CDx, the first breakthrough-designated, next generation sequencing (NGS)-based in vitro diagnostic test that can detect genetic mutations in 324 genes and two genomic signatures in any solid tumor type.
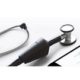
Eko Core is the only stethoscope on the market to wirelessly stream heart sounds to a HIPAA-compliant smartphone app and the first to integrate heart sounds directly into the patient’s electronic health record (EHR), paving the way for clinicians to better address a cardiovascular disease crisis that affects 1 in 4 people worldwide.

We conclude our Celebrating Women in Healthcare Month by saluting Dr. Margaret Hamburg as she resigns after six years of serving as the Commissioner of the U.S. Food and Drug Administration.










